How many milliliters of a 45 M ammonium hydroxide solution contain 0534 moles of solute. 12 mL 2 mL 4 mL 120 mL.

Ethane Structure Uses Formula Video Lesson Transcript Study Com
D Ethane reacts with chlorine in the presence of ultraviolet light to form a mixture of products.
. 1 Bond broken Bond fission A heterolytic B 1 heterolytic C homolytic. This mechanism is similar to that which occurs when methane is chlorinated. Draw the structure for 2-amino-3-ethyl-1-chloroheptane.
What is the molar. C2H6 Cl2 C2H5Cl HCl State the conditions and outline a mechanism for this reaction. Ammonium NH 4 ions in which one or more hydrogen atoms are replaced with alkyl groups are named in a manner analogous to that used for simple amines.
Handbook of textile fibre structure i. Cl 2 o 2Cl Identify the type of bond broken and the type of bond fission occurring in this step. 37 Full PDFs related to this paper.
The overall equation for the reaction of ethane to form chloroethane is given below. I In the initiation step chlorine molecules are converted into radicals. Chlorination of ethane follows a free-radical substitution mechanism.
Solution for A sample containing an unknown concentration of FeCl3 is measured to have an absorbance of 043 at 334 nm in a 10 cm cuvet. This is a form of energy that is related to movement. Temperature is a physical parameter that indicates how hot or cold something isIt is required especially for the calculation of the average kinetic energy of the particles in an item.
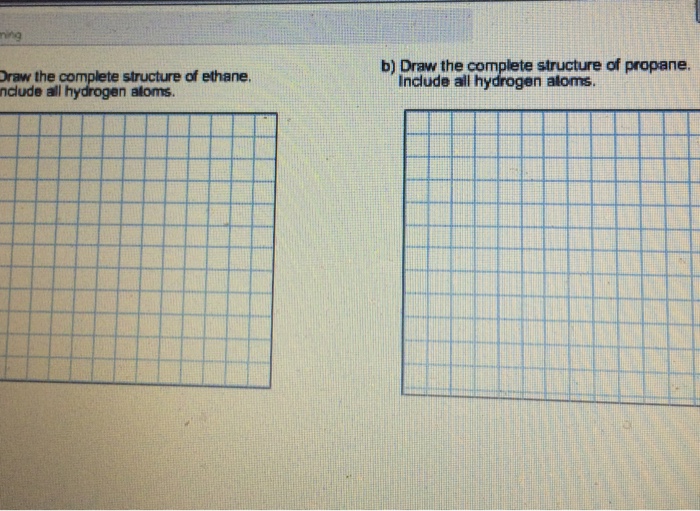
Solved Draw The Complete Structure Of Ethane Include All Chegg Com
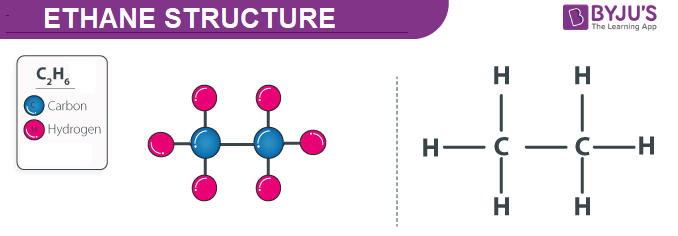
Ethane Structure Properties And Uses Of C2h6

Ethane Formula Structural And Chemical Formula Of Ethane

Draw The Electronic Dot Structure Of Ethane Molecule C2h6
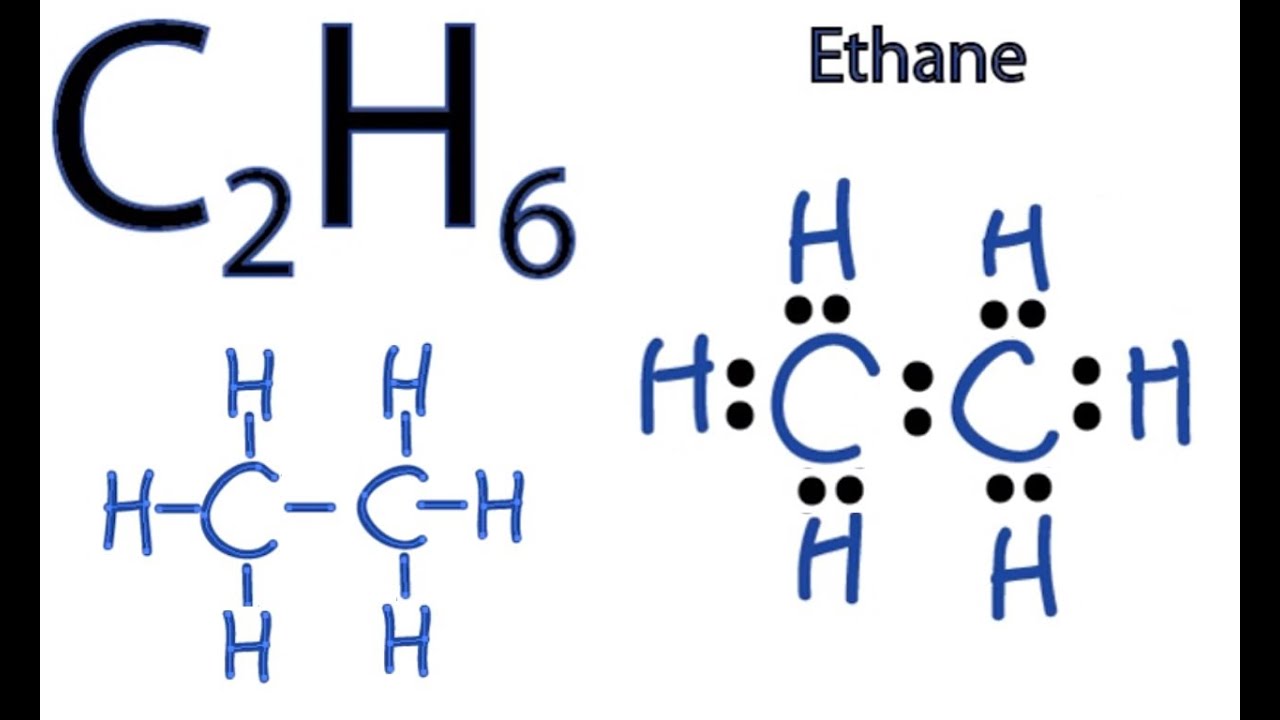
C2h6 Lewis Structure How To Draw The Dot Structure For C2h6 Youtube

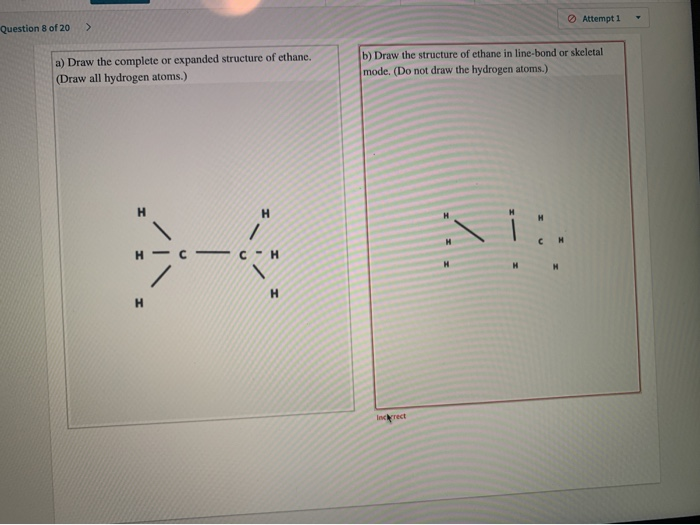
Solved Attempt 1 Question 8 Of 20 A Draw The Complete Or Chegg Com
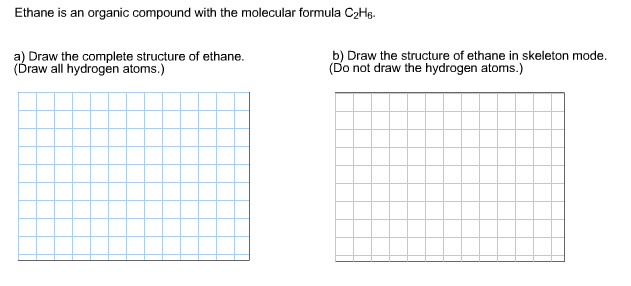
Solved Ethane Is An Organic Compound With The Molecular Chegg Com
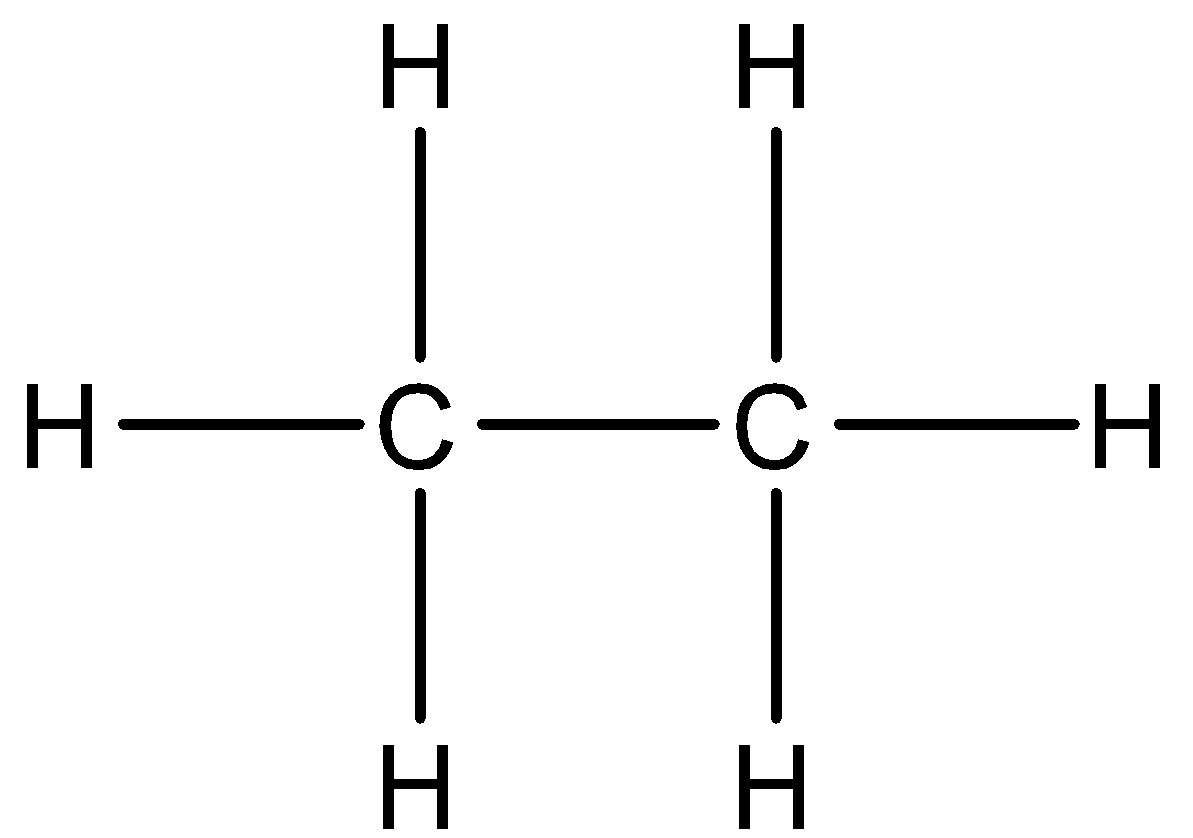
Draw The Structural Formula Of Ethane Draw Electrondot Class 11 Chemistry Cbse
0 comments
Post a Comment